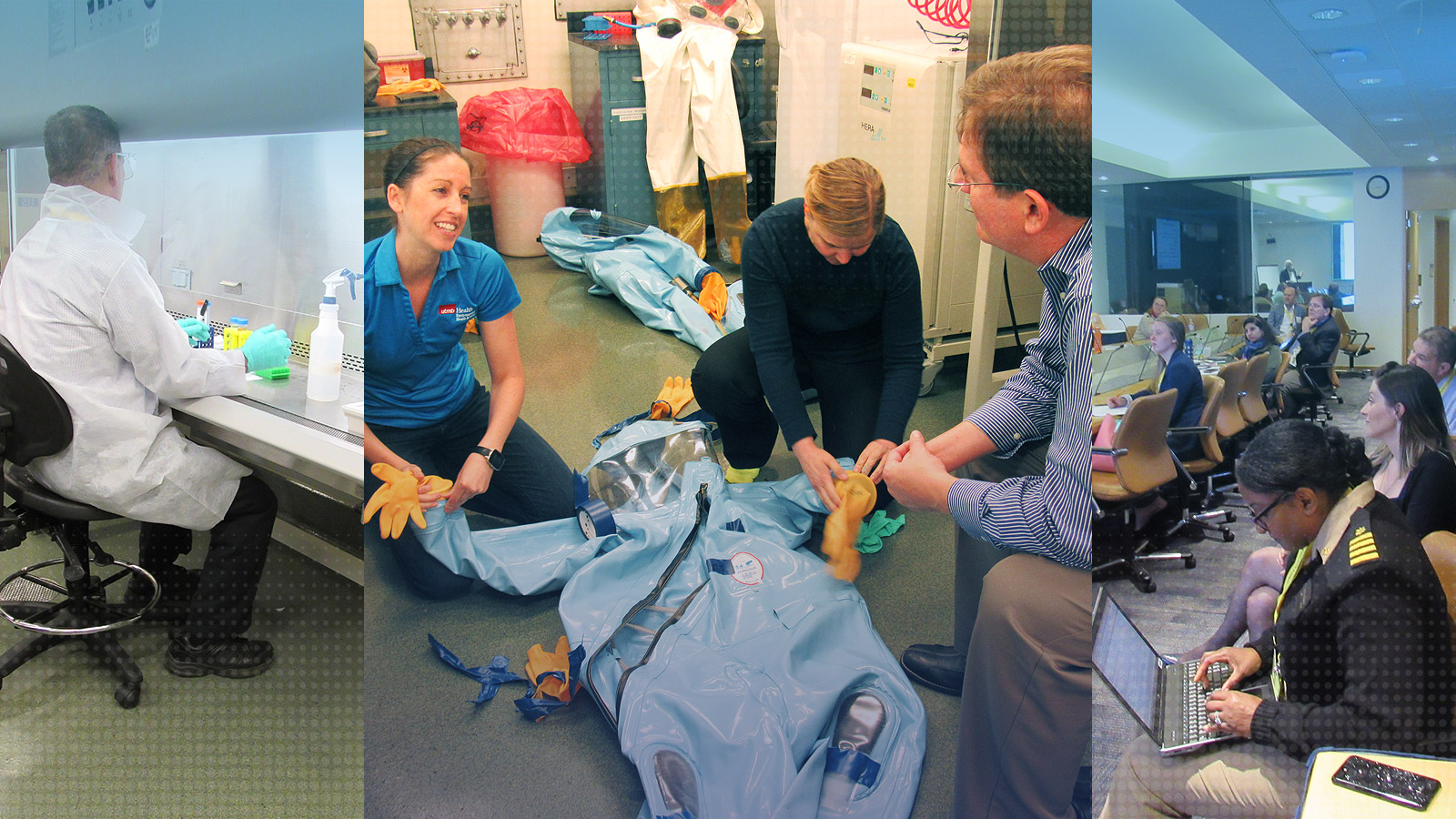
To help address challenges associated with ensuring data quality and integrity in regulated studies conducted in BSL-4 laboratories to support MCM development, FDA and UTMB collaborate to provide an annual training course on how to meet GLP requirements in BSL-4 facilities
administrator
Related Articles
27th Meeting of the Society of Hair Testing…
- December 13, 2025
New Metabolites of Methyldienolone by In Vitro Human Liver…
- December 12, 2025
A First Report of the Misuse of a…
- December 12, 2025
